Par Nicholas Harris
Le 01/01/2016
Le 01/01/2016
Headspaces discussions at Cetie
It is a fact of life that, with a few exceptions, substances expand when the temperature rises. Moreover, as a general rule, liquids expand much more than solids for the same rise in the temperature. This is the reason why it is necessary to take account of what happens when the temperature rises after closing, when putting liquids in rigid containers, especially since the forces generated by the thermal expansion of a liquid or solid are effectively irresistible. An empty volume, or headspace, must be left on top of the product inside the closed bottle. Let’s recap some of the basic principles.
The temperature of a product contained in a closed bottle can vary:
either in a controlled manner, during pasteurisation or sterilisation heat treatments, or in a less controlled manner, during transportation and storage, for example in a truck or the boot of a car parked in direct sunlight. When the temperature rises, the liquid expands and compresses the gas in the headspace. Therefore, the volume of the headspace must be determined according to the maximum expected temperature, in order to limit the resulting overpressure and to keep the closure airtight and prevent the risk of the bottle bursting. This is all the more critical, because the pressure increases very quickly when the liquid expands significantly in comparison with the volume of the headspace.
Since the regulations require the nominal filling volumes to be defined at 20°C, the headspaces are also defined at this temperature, irrespective of the actual filling temperature. The volume of the headspace is usually expressed as a percentage of the nominal volume of product (at 20°C), because its effects are essentially proportional to the volume.
Figure 1 below illustrates this phenomenon by showing the theoretical increase of the relative pressure in a glass bottle filled with water at 20°C and at atmospheric pressure, for various values of the headspace. In this example, we will make the simplifying assumption that there are no exchanges between the gas in the headspace and the liquid, in other words, that the level of the liquid simply acts like a piston. The figure also shows (the vertical “closure contact” lines) the temperature at which the liquid completely fills the headspace for values of 1%, 2% and 3%. In these cases, either the closure or the bottle would inevitably yield.
Since the regulations require the nominal filling volumes to be defined at 20°C, the headspaces are also defined at this temperature, irrespective of the actual filling temperature. The volume of the headspace is usually expressed as a percentage of the nominal volume of product (at 20°C), because its effects are essentially proportional to the volume.
Figure 1 below illustrates this phenomenon by showing the theoretical increase of the relative pressure in a glass bottle filled with water at 20°C and at atmospheric pressure, for various values of the headspace. In this example, we will make the simplifying assumption that there are no exchanges between the gas in the headspace and the liquid, in other words, that the level of the liquid simply acts like a piston. The figure also shows (the vertical “closure contact” lines) the temperature at which the liquid completely fills the headspace for values of 1%, 2% and 3%. In these cases, either the closure or the bottle would inevitably yield.
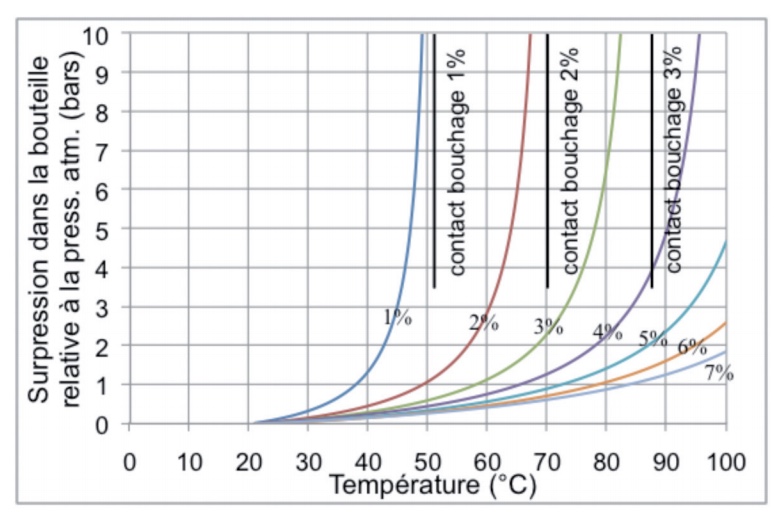
Figure 1 Theoretical increase of the pressure in a glass bottle filled with water according to the temperature and with different headspace volumes
Even if the increase in the pressure versus the volume of the headspace depends, in particular, on the composition of the product and the possibility for the gases to dissolve in the liquid, the overall shape of these curves will always be the same, showing that the rise in pressure depends greatly on the volume of the headspace.
Therefore, leaving sufficient headspace is a very important factor in order to guarantee the quality of commercial products and to avoid losses of significant quantities. This is especially true when the closure system is not designed to withstand overpressure, such as the ROPP caps for still products that are not usually intended for pressures higher than 1 bar.
The composition of the product affects its coefficient of expansion. In particular, alcohol has a significantly higher coefficient of volume expansion than water, resulting in faster rises in pressure. This is illustrated in Figure 2, using the same theoretical basis as Figure 1, and by comparing the behaviour of water with that of a mixture of water and 20% ethanol by volume, with different headspace volumes.
Therefore, leaving sufficient headspace is a very important factor in order to guarantee the quality of commercial products and to avoid losses of significant quantities. This is especially true when the closure system is not designed to withstand overpressure, such as the ROPP caps for still products that are not usually intended for pressures higher than 1 bar.
The composition of the product affects its coefficient of expansion. In particular, alcohol has a significantly higher coefficient of volume expansion than water, resulting in faster rises in pressure. This is illustrated in Figure 2, using the same theoretical basis as Figure 1, and by comparing the behaviour of water with that of a mixture of water and 20% ethanol by volume, with different headspace volumes.
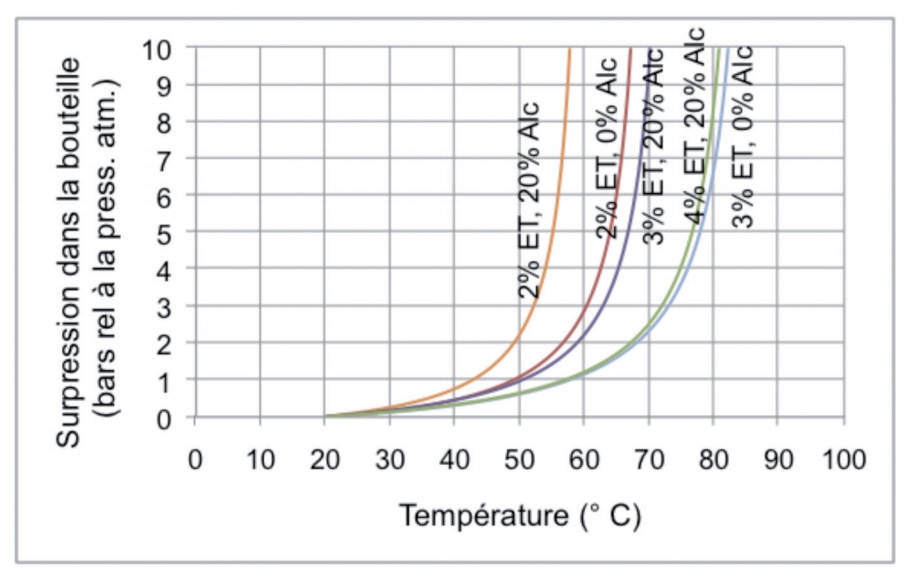
Figure 2 Theoretical increase in the pressure of water and of a mixture of water and 20% ethanol by volume in a glass bottle according to the temperature and with different headspace volumes
We can see how the alcohol brings about the increase in pressure at significantly lower temperatures. By way of example, a mixture containing 20% of alcohol by volume would require a headspace of almost 3% in order to produce the same increase in pressure as that observed for water with a 2% headspace. Sugar is another common ingredient found in drinks that has a similar effect, but to a lesser extent.
"In practice, the recommendations applying to headspaces must be based on the industrial experience gained with each product family."
In reality, the situation is clearly more complex, because the gas in the headspace always interacts with the liquid, either physically, if the gas is inert, like nitrogen, or chemically, if there is a reaction with the product, like that between carbon dioxide and water to form carbonic acid. Flushing the headspace with nitrogen or carbon dioxide during bottling prevents this risk for products sensitive to oxidation. Even if, at equilibrium, these interactions tend to reduce the pressures reached to within certain limits, they involve kinetic processes that may work much more slowly than thermal expansion and thus generate transient pressure increases that would follow the figures shown above.
In practice, the recommendations applying to headspaces must be based on the industrial experience gained with each product family. This takes account in particular of the maximum temperatures observed in transport and storage, which are estimated at between 40°C and 50°C. The catalogue bottle models reflect this practice, and in particular the filling height as a measuring recipient takes the necessary headspace into consideration. But it is the bottler’s responsibility to leave the prescribed headspace, while taking account of the temperature of the product when the bottle is filled.
In practice, the recommendations applying to headspaces must be based on the industrial experience gained with each product family. This takes account in particular of the maximum temperatures observed in transport and storage, which are estimated at between 40°C and 50°C. The catalogue bottle models reflect this practice, and in particular the filling height as a measuring recipient takes the necessary headspace into consideration. But it is the bottler’s responsibility to leave the prescribed headspace, while taking account of the temperature of the product when the bottle is filled.
By N. Harris Cetie General Secretary
Published in Liquides & Conditionnement N°381 (January-February-March 2016)
PDF (FR) :
- Liquides & Conditionnement N°381 (FR) Tags : , , ,
Commentaires
Aucun commentaire
Ajouter commentaire
Vous devez être connecté pour pouvoir saisir un commentaire.
Recherche



